The basics of asphalt used in roofing
How to make roofing asphalt
By Mike Anderson, P.E.
In the first article in this series, we discussed the history of asphalt roofing and the constituents of composition roofing systems (shingles). In the second article, we discussed the properties of a good roofing asphalt for steep-slope applications (shingles). In this third article, we will discuss how to make a roofing asphalt.
As a note for the reader, there likely will not be a fourth article because an unwritten rule for authors states that if producing a multi-part series it must be in the form of a trilogy or seven-part series. For reference, see J.R.R. Tolkien (Lord of the Rings), J.K. Rowling (Harry Potter), C.S. Lewis (Chronicles of Narnia), Suzanne Collins (The Hunger Games) and Stephen King (The Dark Tower). Even George Lucas is sort of following this script by creating a trilogy of trilogies (Star Wars). Peter Jackson is as well by turning “The Hobbit” into a three-part movie. Having said that, I guess I better get to this article before “The Hobbit: The Battle of the Five Armies” is released.
Let’s start at the very beginning
According to Julie Andrews it is a very good place to start. Roofing asphalts are made by first starting with a flux – a low-viscosity asphalt – and then subjecting the flux to an oxidation process to achieve the desired properties. Typical asphalt fluxes used for roofing manufacturing would be characterized as an AC-2.5 or PG 46-xx material in paving terms. Without additional processing these asphalts would be too soft for typical paving applications.
It is not unusual for a manufacturer to use blends of fluxes to get the desired properties after processing. Sometimes blends are used to upgrade a flux that is considered by the manufacturer to have less than optimal physical or chemical properties. Other times, blends are used because of availability. Additives and catalysts – such as polyphosphoric acid (PPA) and ferric or ferrous salts – may also be used during the oxidation process to help the finished product reach its desired set of properties.
Oxidation (air blowing)
Once the formulation of the material has been selected, the blend is ready to go through the oxidation, or air blowing, process. Air blowing is the process of passing air through heated asphalt to raise the softening point of the asphalt while maintaining much of its flexibility at lower temperatures. In a conventional batch oxidation process, the flux enters a pre-heater at approximately 121-149°C (250-300°F) and is heated to and maintained at 232-260°C (450-500°F) entering the oxidation still. There it stays, with air bubbling through it for several hours, until the softening point has been raised to approximately 100- 105°C (212-220°F) and the penetration value has dropped into the range of 15-23 dmm at 25°C (77°F). The conventional batch oxidation process is shown in Figure 1. The continuous oxidation process is similar except that the product moves from one still to the next during oxidation until the product has reached its desired properties.
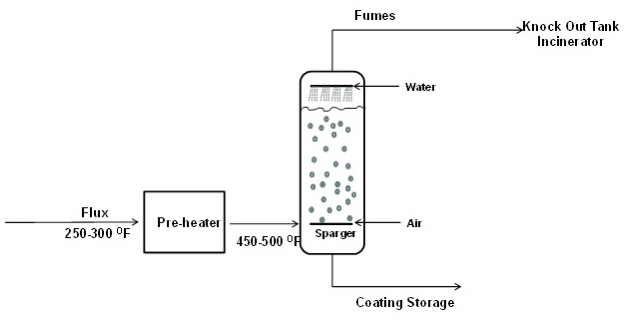
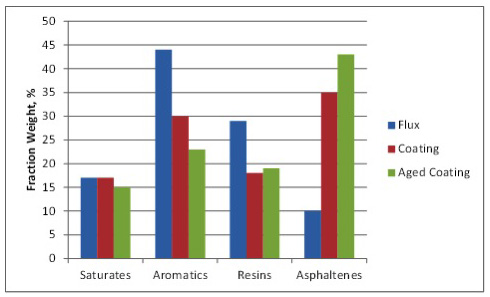
Asphalt oxidation is a complex controlled exothermic reaction process involving the reaction and rearrangement of asphalt molecules in the presence of oxygen at elevated temperatures (such as in a blow still or rooftop). The process includes dehydrogenation, aromatization, oxygen uptake, and a loss of volatile material. The average molecular size of the asphalt molecules increases as the components of asphalt – generally referred to as SARA fractions – change proportions. Saturates (paraffins, waxes designated as “S”) change very little as they are fairly unreactive. Aromatics (naphthene aromatic rings designated as “A”) change to Resins (polar aromatic rings designated as “R”) which, in turn, change to Asphaltenes (large, polar aromatic rings) during oxidation. Changes in the SARA fractions as a result of oxidation are shown in Figure 2. Note that the aged coating exhibits additional oxidation in the form of reduced aromatics and increased asphaltenes compared to the coating.
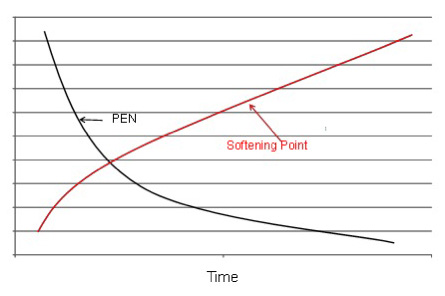
The oxidation process and conversion of aromatic components to asphaltenes causes the softening point and viscosity to increase and the penetration to decrease as the time in the oxidation still increases (Figure 3 below.)
Not all oxidation processes are the same as there are several parameters that can have an effect on the final oxidized product. The size of the oxidation still, specifically the height/diameter ratio, is important to control the surface area of the air bubbles. The size of the air bubbles can be affected by the sparger size. Smaller bubbles have an increased surface area, allowing for more reaction. Likewise, the volume of air flow controls the residence time of the bubbles which affects the reaction.
Like everything else asphalt-related, temperature is a key parameter. While the oxidation process typically occurs around 260°C (500°F), the temperature can be increased to reduce the oxidation time and harden the final product (coating) by increasing the softening point/penetration ratio. Conversely, decreasing the temperature will increase the oxidation time and soften the final product (coating) by decreasing the softening point/ penetration ratio. Either technique may be employed by the manufacturer depending on the specific properties of the flux, or blend of fluxes, being used.
For safety reasons it is important to remember that during the oxidation process the asphalt should not be heated to an elevated temperature that approaches its flash point. As a matter of caution, the oxidation temperature that is used should be at least 28°C (50°F) lower than the flash point (Cleveland Open Cup or COC) of the asphalt being oxidized.
Finally, catalysts like ferric and ferrous salts may be used to impact the oxidation process and affect the properties of the finished product. The use of these catalysts may improve the flexibility and weathering resistance and increase the penetration value for a specific softening point, but the improvement in properties is very much crude-dependent. Special handling and control equipment is needed when using these catalysts. Although not considered a “true” catalyst, polyphosphoric acid (PPA) may also be used to a similar effect as the ferric and ferrous salts.
The end game
The final chemical and physical properties of a roofing asphalt is dependent on a number of things including the source (or sources) of flux used, the addition of any catalysts, and the oxidation process to name a few. A manufacturer needs to understand the impacts that all of these factors, and more, have on physical properties like resistance to flow, flexibility and resistance to weathering. That knowledge is what gives the customer the confidence to use asphalt shingles and expect that they’ll perform.
Acknowledgments and references
The information in this article was derived from the “Roofing 101” presentation conducted at the Asphalt Institute’s Annual Meeting in 2012 and AI’s “MS-26 Asphalt Binder Handbook.” Special thanks go to the members of the Asphalt Institute Roofing Technical Advisory Committee – specifically Andrew Ford, Mike Franzen, Greg Malarkey, Andrew Parker, Ken Grzybowski and Keith Stephens – for their input.
Anderson is the Director of Research and Laboratory Services at the Asphalt Institute.